Image: CHASE
The newest breakthrough in sustainable energy could create fuel out of thin air.
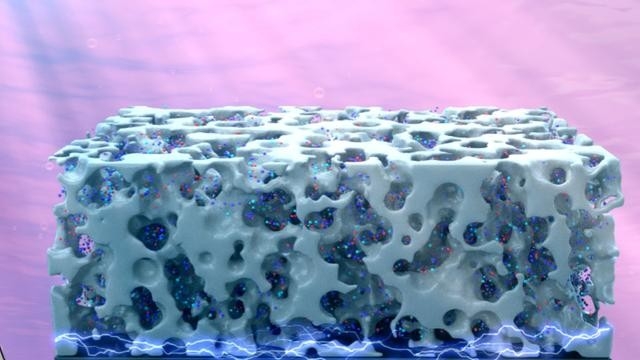
The Department of Energy Center for Hybrid Approaches in Solar Energy to Liquid Fuels implemented a revolutionary strategy that utilized a high-surface-area silicon material to generate liquid solar fuel from sunlight, carbon dioxide, and water.
While previous efforts have successfully used silicon-based photoelectrodes to create liquid solar fuel, CHASE, which includes researchers from six universities and a laboratory, found that a three-dimensional silicon format in the shape of micropillars increased the efficiency of the system and yield of the desired product.
The scientists ran two separate experiments that placed a different type of catalyst on the silicon photoelectrode, which helped them analyze the experiment at a molecular level. The catalyst absorbs sunlight to trigger chemical reactions that convert CO2 in the presence of water into fuel — a process not unlike photosynthesis.
They conducted the first trial using cobalt as the catalyst and then replaced it with rhenium. The former produced methanol with an improved current density, while the latter demonstrated more "durability and selectivity" as it transformed carbon monoxide into methanol.
The International Renewable Energy Agency believes that increased dedication to and reliance on green methanol production — such as a Chinese natural gas company's efforts to convert kitchen waste into methanol for container ships using the fuel — could prevent 1.6 billion tons of CO2 pollution annually.
Both experiments delivered promising results for the future of renewable energy and could help heavy industries and applications that typically rely on dirty energy sources transition to cleaner alternatives.
Source: MIT Technology Review/TCD
Image: CHASE